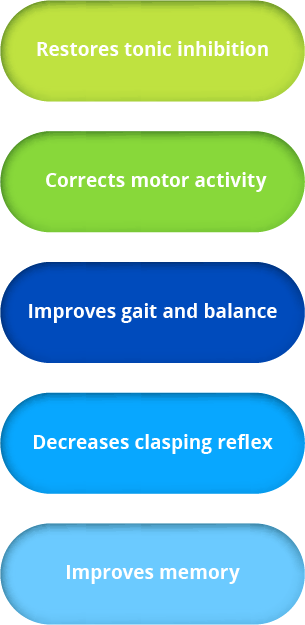
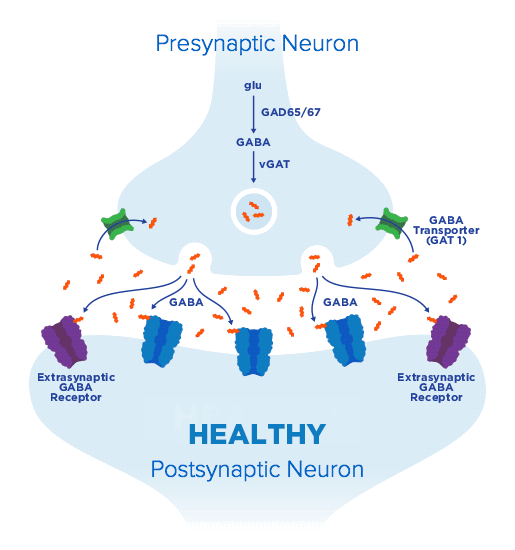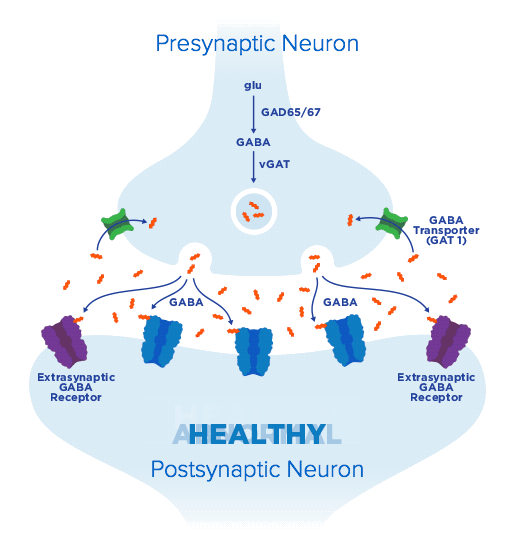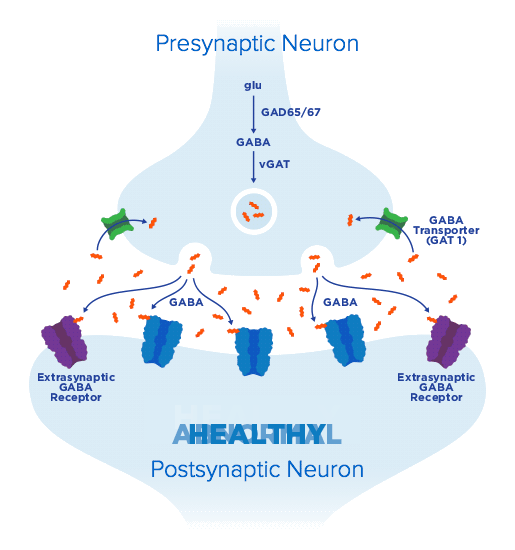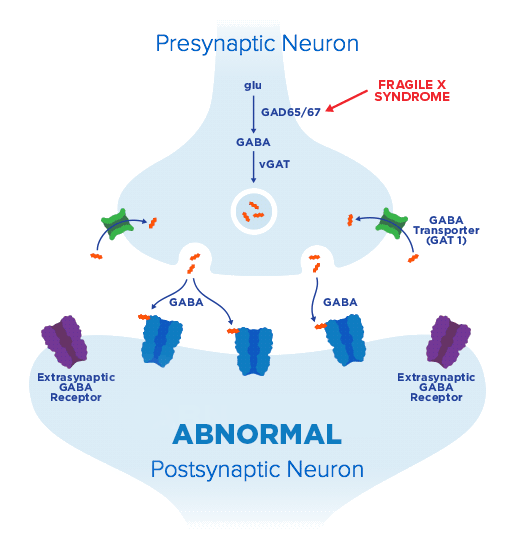
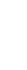our science
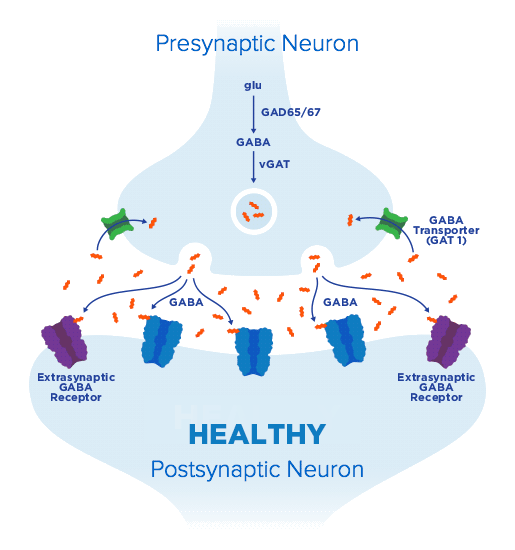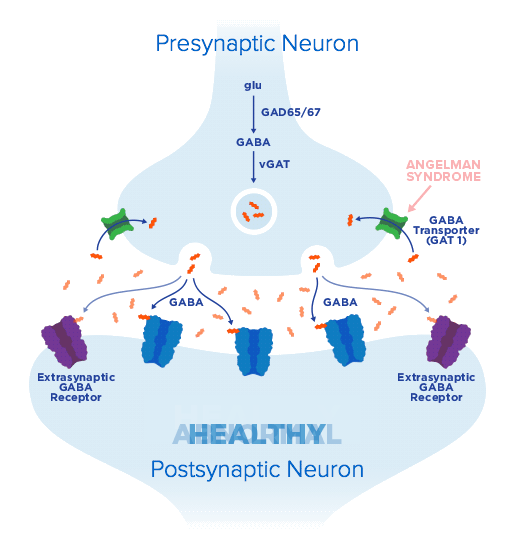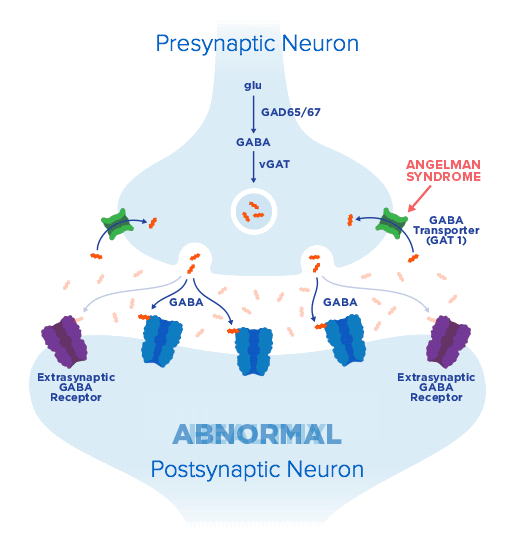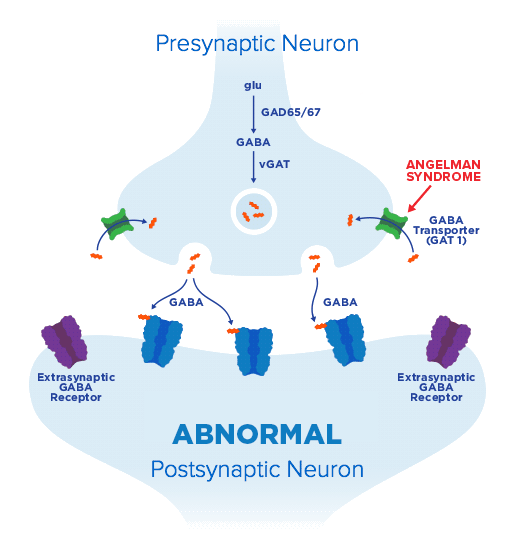
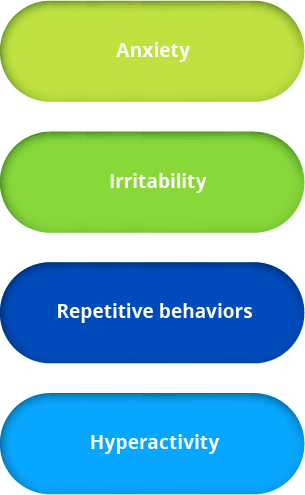
From clinical potential to meaningful therapies—Ovid Therapeutics is developing lifechanging therapies based on our deep understanding of key biological pathways and their central role in rare neurological diseases. We seek to develop medicines using novel and clinically relevant endpoints, to capture the real-world ways patients benefit from addressing underlying disorder pathology.